2.2 Subatomic Particles
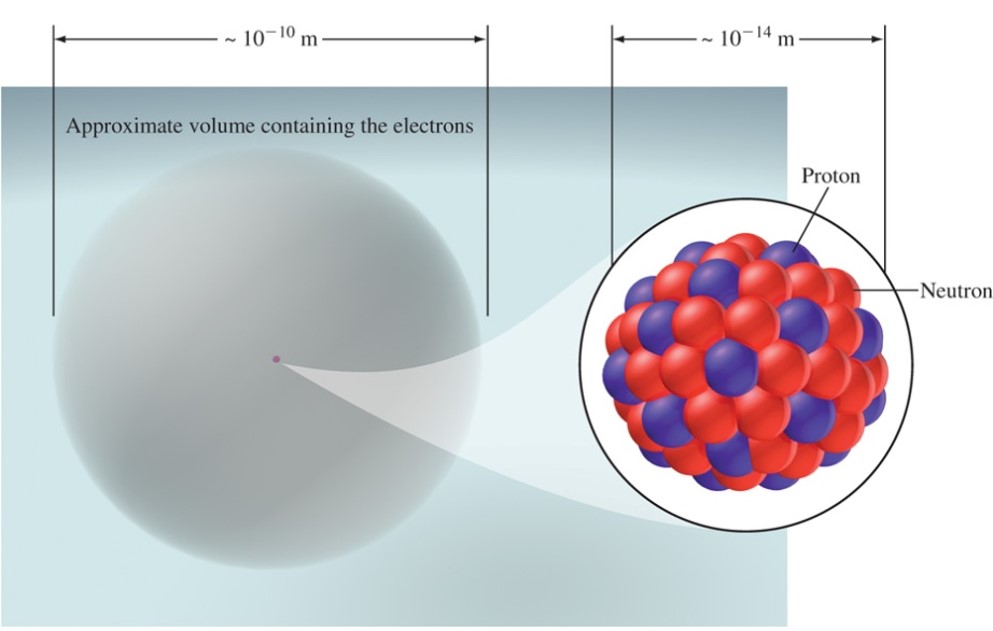
Protons are positively charged particles found in the nucleus. Neutrons are electronically neutral particles found in the nucleus. Neutrons are slightly larger than protons. Electrons are negatively charged particles distributed around the nucleus.
| Particle | Mass (g) | Mass (amu) | Charge (C) | Charge Unit |
|---|---|---|---|---|
Electron |
9.10938×10–28 |
5.534×10–4 |
–1.6022×10–19 |
–1 |
Proton |
1.67264×10–24 |
1.0073 |
+1.6022×10–19 |
+1 |
Neutron |
1.67493×10–24 |
1.0086 |
0 |
0 |
Practice
Which of the following is true about protons and neutrons?
A. They both have a charge.
B. They have approximately the same mass.
C. They are unstable with respect to radioactive decay.
D. They are found in the same region of space around the nucleus as the electron
Solution
B
Practice
Which experiment can be linked to Millikan?
A. Photographic plate fogged with Uranium – discovering radioactivity
B. Oil drop experiment – charge of an electron
C. Shooting alpha particles at gold foil – structure of the atom
D. Cathode ray tube – mass of an electron
Solution
B