7.1 Practice Problems
Attempt these problems as if they were real exam questions in an exam environment.
Only look up information if you get severely stuck. Never look at the solution until you have exhausted all efforts to solve the problem. Having to look up information or the solution should be an indicator that the previous layers (1–5) in the Structured Learning Approach have not been mastered.
Which of the following statements is incorrect?
- A spectator ion is one that is in its aqueous form on one side of the reaction arrow, but in a solid form on the other side in a complete ionic equation.
- If you mix two salt solutions and notice a solid starting to form from the
solutions, gathering at the bottom of the mixture flask,
you can call the solid the “precipitate”.
- A net ionic equation does not include spectator ions.
- (aq) denotes a species dissolved in aqueous solution.
- Hydroxide ions often form insoluble compounds, but some hydroxides (like sodium hydroxide) are soluble in water.
Solution
Answer: A
Concept: Reactions and reaction types
Option A is incorrect because a spectator ion will have the same phase label on both sides of the reaction.
In which of the below species does nitrogen have the highest (most positive) oxidation number?
- NaNO3
- N2
- NH3
- HNO2
- NO2–
Solution
Answer: A
Concept: Oxidation states
N has an oxidation state of:
- +5
- 0
- +3
- +3
- +3
What is the coefficient found in front of the H2O in the balanced combustion reaction equation between C4H10 and molecular oxygen?
- 5
- 10
- 13
- 26
- none of these
Solution
Answer: B
Concept: Balancing equations
A combustion reaction produces CO2 and H2. Write the reaction and balance.
\[\begin{align*} \mathrm{C_4H_{10}} + \mathrm{O_2} &\longrightarrow \mathrm{CO_2} + \mathrm{H_2O} \\[2ex] 2\mathrm{C_4H_{10}} + 13\mathrm{O_2} &\longrightarrow 8\mathrm{CO_2} + 10\mathrm{H_2O} \end{align*}\]
Identify the reaction below that corresponds to a decomposition reaction.
- 2NaClO3 → 2NaCl + 3O2
- BaCl2 + Na2SO4 → BaSO4 + 2NaCl
- CH4 + 2O2 → CO2 + 2H2O
- Mg + CaCl2 → MgCl2 + Ca
- 2H2 + O2 → 2H2O
Solution
Answer: A
Concept: Reaction types
Identify the reaction below that corresponds to a decomposition reaction
- Zn(NO3)2 + Ba(CH3COO)2 →
- Al(s) + ZnCl2 →
- Zn(s) + AlCl3 →
- KNO3 + NaCH3COO →
- none of these will produce products
Solution
Answer: B
Concept: Reaction types
17.84 mL of 0.323 M FeCl3 and 10.65 mL of 0.752 M NaOH are reacted. How many moles of NaCl(aq) are produced if the following reaction goes to completion?
\[\mathrm{FeCl_3}(aq) + 3\mathrm{NaOH}(aq) \longrightarrow \mathrm{3NaCl}(aq) + \mathrm{Fe(OH)_3}(s)\]- 0.2001
- 0.0173
- 0.0940
- 0.0080
- 0.3510
Solution
Answer: D
Concept: Limiting reactant
First, make sure the reaction is balanced.
Find the theoretical yield (in mol) of a product if each reactant were to completely react. Here I choose NaCl to be the target product.
\[\begin{align*} \mathrm{FeCl_3}: ~n_{\mathrm{NaCl}} &= \left ( \dfrac{17.84~\mathrm{mL}}{10^3~\mathrm{L}} \right ) \left ( \dfrac{0.323~\mathrm{mol~FeCl_3}}{\mathrm{L}} \right ) \left ( \dfrac{3~\mathrm{mol~NaCl}}{1~\mathrm{mol~FeCl_3}} \right )\\[2ex] &= 0.0173~\mathrm{mol~NaCl} \\[4ex] \mathrm{NaOH}: ~n_{ \mathrm{NaCl}} &= \left ( \dfrac{10.65~\mathrm{mL}}{10^3~\mathrm{L}} \right ) \left ( \dfrac{0.752~\mathrm{mol~NaOH}}{\mathrm{L}} \right ) \left ( \dfrac{3~\mathrm{mol~NaCl}}{3~\mathrm{mol~NaOH}} \right )\\[2ex] &= 0.00800~\mathrm{mol~NaCl} \end{align*}\]
NaOH is the limiting reactant. Therefore, if the reaction goes to completion, only 0.00800 mol of NaCl would be produced.
What are the spectator ions for the following reaction:
\[\mathrm{HCl} + \mathrm{Ca(OH)_2} \longrightarrow \mathrm{CaCl_2} + \mathrm{H_2O}\]- Proton
- H2O
- Hydroxide
- Calcium and chloride
- Proton and hydroxide
Solution
Answer: D
Concept: Ionic and net ionic equations
When writing out the full ionic equation for this reaction, Ca2+ and Cl– appears on both sides and can be cancelled out meaning they are spectator ions.
What volume (in mL) of 0.223 M Mg(OH)2 is required to neutralize 26.7 mL of 1.2 M HI?
- 88.93
- 0.223
- 71.84
- 0.072
- 52.36
Solution
Answer: C
Concept: Reaction stoichiometry
Recognize that a neutralization reaction will occur where the hydroxide (OH–) of the magnesium salt will react with the strong acid (HI) in a 1:2 ratio due to every Mg(OH)2 giving 2OH–.
Determine the number of moles of HI present and divide by 2 to find the moles of Mg(OH)2 needed for a complete neutralization.
\[\begin{align*} n_{\mathrm{HI}} &= \left ( \dfrac{26.7~\mathrm{mL}}{} \right ) \left ( \dfrac{\mathrm{L}}{10^3~\mathrm{mL}} \right ) \left ( \dfrac{1.2~\mathrm{mol}}{\mathrm{L}} \right ) \\[2ex] &= 0.03204~\mathrm{mol~HI} \\[4ex] n_{\mathrm{Mg(OH)_2}} &= \left ( \dfrac{0.03204~\mathrm{mol~HI}}{} \right ) \left ( \dfrac{1~\mathrm{mol~Mg(OH)_2}}{2~\mathrm{mol~HI}} \right )\\[2ex] &= 0.01602~\mathrm{mol~Mg(OH)_2} \end{align*}\]
Next, determine the volume of Mg(OH)2 needed.
\[\begin{align*} V_{\mathrm{Mg(OH)_2}} &= \left ( \dfrac{0.01602~\mathrm{mol~Mg(OH)_2}}{} \right ) \left ( \dfrac{\mathrm{L}}{0.223~\mathrm{mol~Mg(OH)_2}} \right ) \left ( \dfrac{10^3~\mathrm{mL}}{\mathrm{L}} \right ) \\[2ex] &= 71.84~\mathrm{mL} \end{align*}\]How many grams of AgCl (143.35 g mol–1) are produced by the unbalanced reaction below if 0.483 moles of silver nitrate are reacted with 0.234 moles nickel(II) chloride?
\[\mathrm{AgNO_3} + ~\mathrm{NiCl_2} ~\longrightarrow ~\mathrm{AgCl} + ~\mathrm{Ni(NO_3)_2}\]- 69.2
- 118.96
- 33.53
- 67.1
- 59.48
Solution
Answer: D
Concept: Limiting reactant
First, make sure the reaction is balanced.
\[2\mathrm{AgNO_3} + \mathrm{NiCl_2} \longrightarrow 2\mathrm{AgCl} + \mathrm{Ni(NO_3)_2}\]
Find the theoretical yield (in mol) of AgCl if each reactant were to completely react.
\[\begin{align*} \mathrm{AgNO_3}: ~n_{\mathrm{AgCl}} &= \left ( \dfrac{0.483~\mathrm{mol~AgNO_3}}{} \right ) \left ( \dfrac{2~\mathrm{mol~AgCl}}{2~\mathrm{mol~AgNO_3}} \right )\\[2ex] &= 0.483~\mathrm{mol~AgCl} \\[4ex] \mathrm{NiCl_2}: ~n_{ \mathrm{AgCl}} &= \left ( \dfrac{0.234~\mathrm{mol~NiCl_2}}{} \right ) \left ( \dfrac{2~\mathrm{mol~AgCl}}{1~\mathrm{mol~NiCl_2}} \right )\\[2ex] &= 0.468~\mathrm{mol~AgCl} \end{align*}\]
NiCl2 is the limiting reactant and can create, in theory, a maximum of 0.468 mol AgCl. Convert moles of AgCl to grams.
\[\begin{align*} m_{\mathrm{AgCl}} &= \left ( \dfrac{0.468~\mathrm{mol}}{} \right ) \left ( \dfrac{143.35~\mathrm{g}}{\mathrm{mol}} \right ) \\[2ex] &= 67.1~\mathrm{g} \end{align*}\]
Which of the following is false for the equation:
\[\mathrm{CH_3OH} + \mathrm{O_2} \longrightarrow \mathrm{CO_2} + \mathrm{H_2O}\]- The reaction is a combustion reaction
- O2 is the oxidizing agent
- The reaction is not balanced
- Oxygen is reduced
- The oxidation state of carbon in CO2 is –4
Solution
Answer: E
Concept: Redox reactions and oxidation states
- The reaction is a combusion reaction since a carbon/hydrogen-containing compound reacts with oxygen gas to produce carbon dioxide and water.
- O2 is the oxidizing agent meaning O2 undergoes reduction and gains electrons (oxygen’s oxidation state decreases in this reaction).
- The reaction is balanced.
- As seen with (b), oxygen undergoes reduction.
- The oxidation state of carbon in CO2 is +4.
If 4.4 mol of A reacts with 5.5 mol of B, which reactant will be the limiting reagent according to the following reaction?
\[\mathrm{2A} + \mathrm{3B} \longrightarrow \mathrm{A_2B_3}\]- A
- B
- Both A and B will completely react
- Not enough information
- A2B3
Solution
Answer: B
Concept: Limiting reactant
Convert moles of each reactant to a chosen product.
\[\begin{align*} \mathrm{A}: ~n_{\mathrm{A_2B_3}} &= \left ( \dfrac{4.4~\mathrm{mol~A}}{} \right ) \left ( \dfrac{1~\mathrm{mol~A_2B_3}}{2~\mathrm{mol~A}} \right )\\[2ex] &= 2.2~\mathrm{mol~A_2B_3} \\[4ex] \mathrm{B}: ~n_{ \mathrm{A_2B_3}} &= \left ( \dfrac{5.5~\mathrm{mol~B}}{} \right ) \left ( \dfrac{1~\mathrm{mol~A_2B_3}}{3~\mathrm{mol~B}} \right )\\[2ex] &= 1.8~\mathrm{mol~A_2B_3} \end{align*}\]
B is the limiting reactant.
Identify the appropriately labeled atoms/molecules (below) for the following reaction:
\[\mathrm{CH_4}(g) + \mathrm{2O_2}(g) \longrightarrow \mathrm{CO_2}(g) + \mathrm{2H_2O}(l)\]Answer Reduced Species Oxidized Species Reducing Agent Oxidizing Agent A
O
C
CH4
O2
B
C
O
O2
CH4
C
O
C
O
C
D
C
O
C
O
E
CH4
O2
O
C
Solution
Answer: A
Concept: Redox reactions
Considering the balanced reaction below, select the oxidation half–reaction for this process:
\[\mathrm{7Ca + 2KMnO_4} \longrightarrow \mathrm{7CaO} + \mathrm{2Mn} + \mathrm{K_2O}\]- K+ → K2+ + e–
- O4– + 2e– → O2–
- Ca → Ca2+ + 2e–
- MnO4– + 7e– → Mn + 4O2–
- Mn2+ → Mn + 2e–
Solution
Answer: C
Concept: Half-reactions
Identify the true statement below.
- Reduction describes the gain of an electron.
- Oxidation describes the gain of an electron.
- Mn(s) → Mn2+ is an example of reduction.
- Mn2+ → Mn(s) is an example of oxidation.
- None of these statements are true.
Solution
Answer: A
Concept: Redox reactions
Using the bond order to rank the strength of the chemical bond between carbon and oxygen in the following compounds with decreasing bond dissociation energy. The Lewis structures of these compounds are shown below.
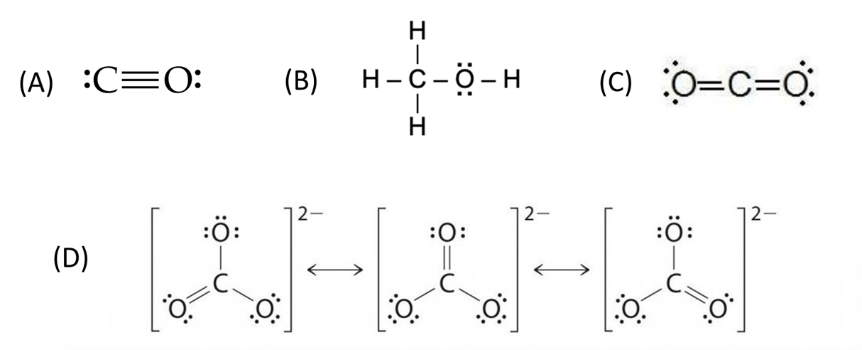
- A > D > C > B
- D > B > C > A
- A > C > D > B
- B > D > C > A
- D > C > B > A
Solution
Answer: C
Concept: Bond energies