3.6 Quantum numbers
Quantum numbers are required to describe the distribution of electron density in an atom (location of the electron). There are three quantum numbers necessary to describe an atomic orbital.
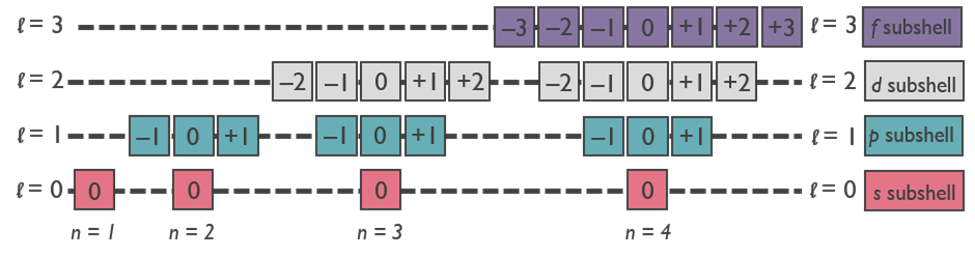
The principal quantum number (n) – designates size
- Larger values of n correspond to larger orbitals (higher energy).
- The allowed values of n are integral numbers: 1, 2, 3 and so forth.
- The value of n corresponds to the value of n in Bohr’s model of the hydrogen atom.
- A collection of orbitals with the same value of n is frequently called a shell.
The angular moment quantum number (l) – describes shape
- The values of l are integers that depend on the value of the principal quantum number
- The allowed values of l range from 0 to n–1. Example: If n = 2, l can be 0 or 1.
| l = | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
Orbital |
s |
p |
d |
f |
A collection of orbitals with the same value of n and l is referred to as a subshell.
The magnetic quantum number (ml) specifies orientation/direction of the orbital.
- The values of ml are integers that depend on the value of the angular moment quantum number: –l,0,+l
The electron spin quantum number (ms) is used to specify an electron’s spin. There are two possible directions of spin. Allowed values of ms are +1/2 and –1/2.