3.4 Heisenberg uncertainty principle
Experiments have shown that electrons do indeed possess wavelike properties:
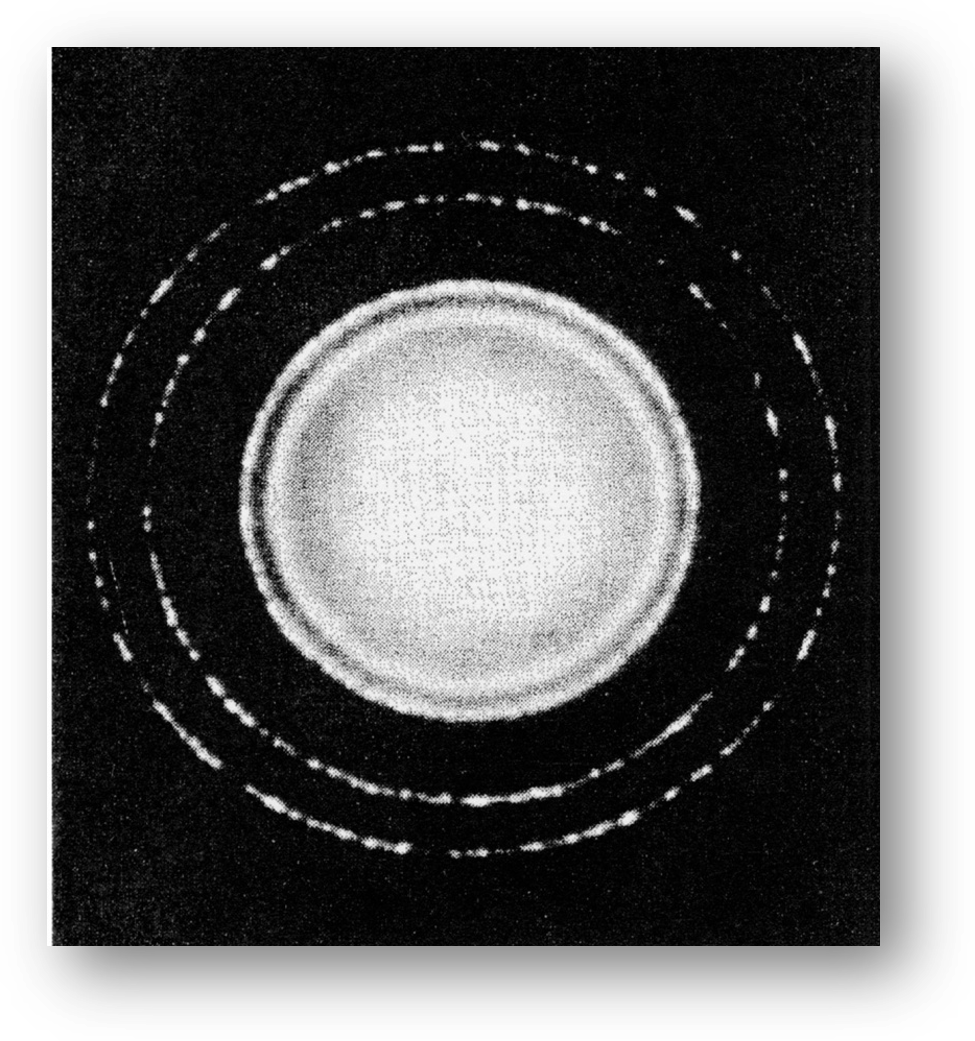
Figure 3.3: Electron diffraction pattern of aluminum foil.
The Heisenberg uncertainty principle states that it is impossible to know simultaneously both the momentum p and the position x of a particle with certainty and can be expressed as
\[\Delta x \cdot \Delta p \ge \dfrac{h}{4\pi}\]
where Δx is the uncertainty in position in meters and Δp is the uncertainty in momentum, as well as,
\[\Delta x \cdot m\Delta v \ge \dfrac{h}{4\pi}\] where Δv is the uncertainty in velocity and m is mass.