2.3 Atomic Symbolism
All atoms can be identified by the number of protons and neutrons they contain. The atomic number (Z) is the number of protons in the nucleus.
- Atoms are neutral, so it’s also the number of electrons.
- Protons determine the identity of an element.
For example, nitrogen’s atomic number is 7, so every nitrogen has 7 protons.
The mass number (A) is the total number of protons and neutrons. Protons and neutrons are collectively referred to as nucleons.
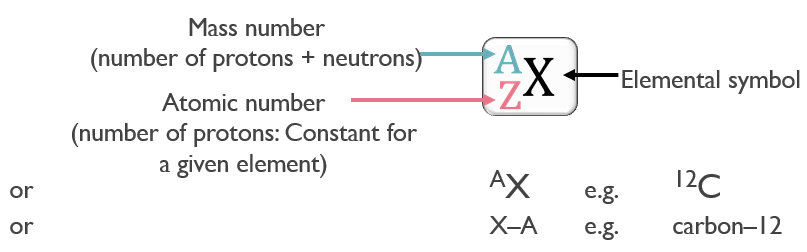
For atoms: we use small units of measurement.
- 1 amu = 1 atomic mass unit = 1/12 the mass of 1 carbon 12 atom
- 1 amu = 1.6605×10–24 g
Practice
Determine the numbers of protons, neutrons, and electrons in each of the following species:
A. 28Si
B. 19F
C. 57Fe
D. carbon–13
Solution
A: 28Si
Z = 14 (14 protons)
A = 28 (28-14 = 14 neutrons)
e– = 14 (equal to the number of protons)
B: 19F
Z = 9 (9 protons)
A = 19 (19-9 = 10 neutrons)
e– = 9 (equal to the number of protons)
C: 57Fe
Z = 26 (26 protons)
A = 57 (57-26 = 31 neutrons)
e– = 26 (equal to the number of protons)
D: carbon-13
Z = 6 (6 protons)
A = 13 (13-6 = 7 neutrons)
e– = 6 (equal to the number of protons)
Practice
Identify the # of protons, neutrons and electrons given the following information:
A. 197Au
B. 48Ti
C. 55Mn
Solution
A: 197Au
p+ = 79
n = 118
e– = 79
B: 48Ti
p+ = 22
n = 26
e– = 22
C: 55Mn
p+ = 25
n = 30
e– = 25
Practice
Which neutral element are the following?
A. 26 protons, 56 neutrons
B. 20 neutrons, 18 electrons, no charge
C. 76 neutrons, 52 protons
Solution
A: Fe
B: Ar
C: Te
Practice
Pick the correct description for 59Co.
A. 59 protons, 27 neutrons
B. 27 protons, 59 neutrons
C. 32 protons, 27 neutrons
D. 27 protons, 32 neutrons
Solution
D
2.3.1 Isotopes
Most elements have two or more isotopes, atoms that have the same atomic number (Z) but different mass numbers (A). This is due to each isotope having a different number of neutrons. Isotopes are atoms of the same element with different A (mass).
- equal numbers of p+
- different numbers of n

Isotopes of the same element typically can exhibit very similar chemical properties.