3.11 Periodic Trends
For all elements in a group (column) on the periodic table exhibit similar chemical behavior. This is attributed to a group having the same number of valence electrons, and those valence electrons being distributed in an equivalent way. This can be seen in the table below:
| Group 1A | Configuration | Group 2a | Configuration |
|---|---|---|---|
Li |
[He]2s1 |
Be |
[He]2s2 |
Na |
[Ne]3s1 |
Mg |
[Ne]3s2 |
K |
[Ar]4s1 |
Ca |
[Ar]4s2 |
Rb |
[Kr]5s1 |
Sr |
[Kr]5s2 |
Cs |
[Xe]6s1 |
Ba |
[Xe]6s2 |
Fr |
[Rn]7s1 |
Ra |
[Rn]7s2 |
For each listed group, all the electrons fill the same orbital shape and have the same number of valence electrons. This similarity is what leads to them reacting similarly. The various groups on the periodic table are labeled according to the image below:
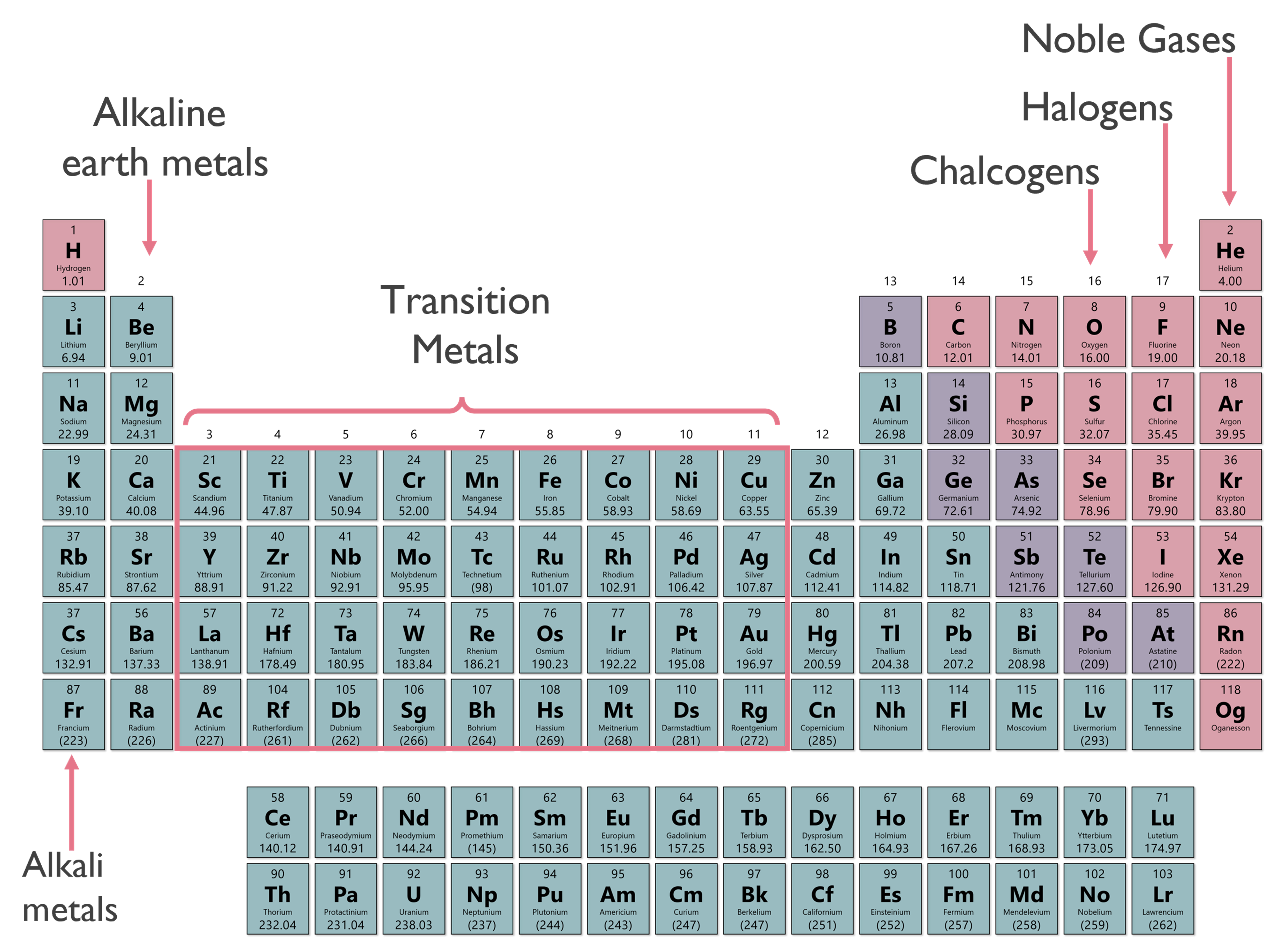
In addition to groups behaving chemically similar, other trends can be established for the periodic table.
First, it is necessary to define a few atomic properties. The atomic radius is defined as the distance between the nucleus of an atom and its valence shell. The atomic radius will increase from right to left and top to bottom across the periodic table, as demonstrated in the image below:
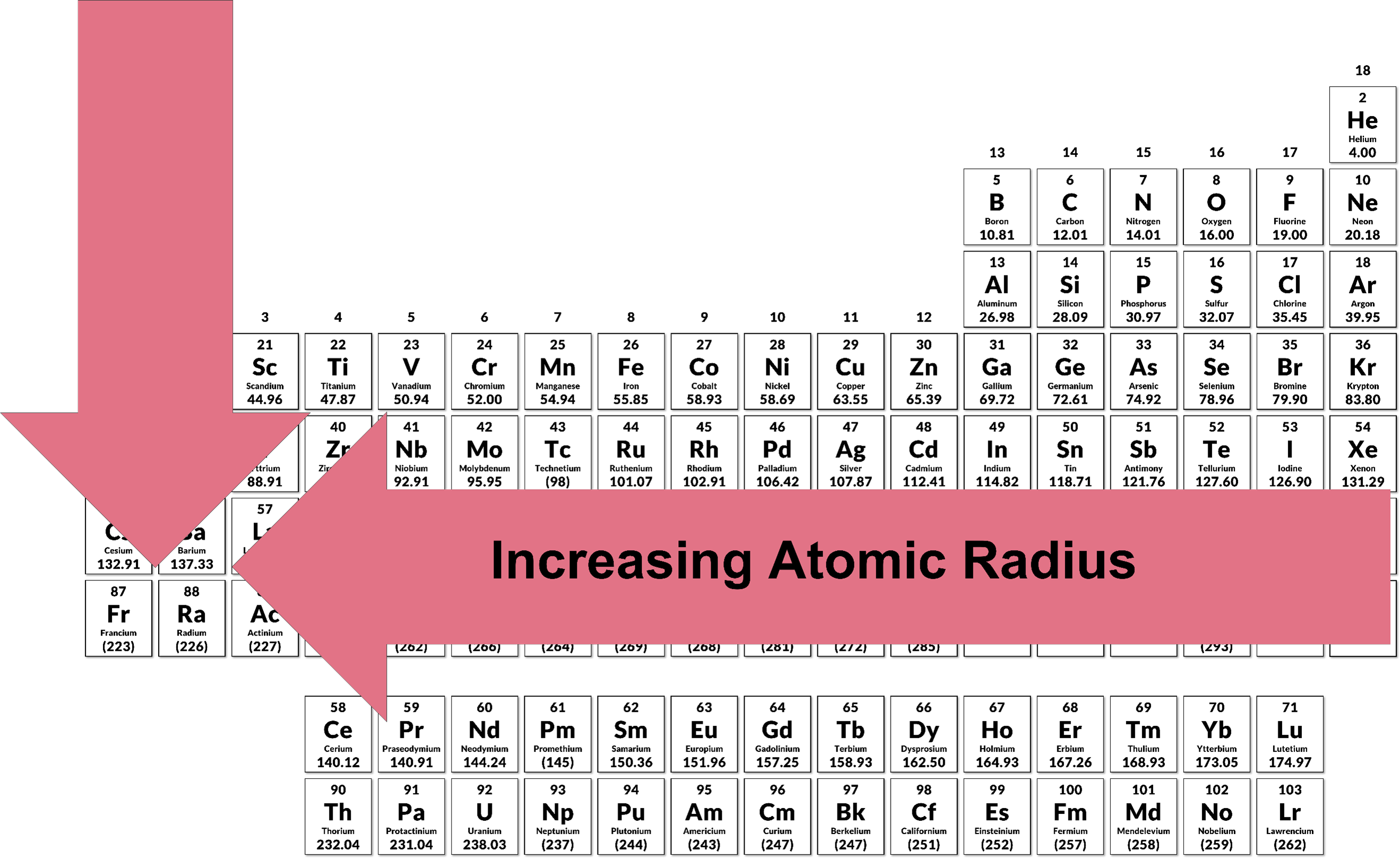
This trend is due to the Effective nuclear charge (Zeff), which is the actual magnitude of positive charge that is “experienced” by an electron in the atom. In a multi-electron atom, electrons are simultaneously attracted to the nucleus and repelled by one another. This interaction between electrons result in what is termed as shielding. Shielding is where an electron is partially shielded from the positive charge of the nucleus by other electrons. This means that the effective nuclear charge increases from left to right because the core electrons remain the same, but the number of protons is steadily increasing as we move from left to right on the periodic table. This can be seen in the table below, as the number of protons (Z) increases, there is an increase in the Zeff.
| Li | Be | B | C | N | O | F | |
|---|---|---|---|---|---|---|---|
Z |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
Zeff |
1.28 |
1.91 |
2.42 |
3.14 |
3.83 |
4.45 |
5.10 |
Since the effective nuclear charge (Zeff) is increasing, the positive charge of the protons is felt more strongly by the electrons. This allows for the electrons to come closer together, with the attraction between electrons and protons being stronger than the repulsion between electrons. Another way to say this, as the electrons feel a stronger attraction to the positive charge of the nucleus, they are pulled closer to the nucleus, decreasing the size of the atomic radius from left to right across the periodic table.
Moving down a column of the periodic table adds another shell to the system, increasing the number of core electrons, which in turns decreases the effective nuclear charge. This decrease leads to increase in atomic radius, as the electrons are feeling less pull from the protons.
In addition to atomic radii, ionic radii should be discussed. When a species is a cation, or positively charged ion, the species has lost an electron. Since there are less electrons to repel, and the effective nuclear charge has remained unchanged, a cation will be smaller than the parent atom. When discussing an anion, or negatively charged ion, the parent atom has gained one or more electrons. This will increase the electron repulsion forces (decreasing effective nuclear charge per electron), which leads to the anion being larger than the parent ion.
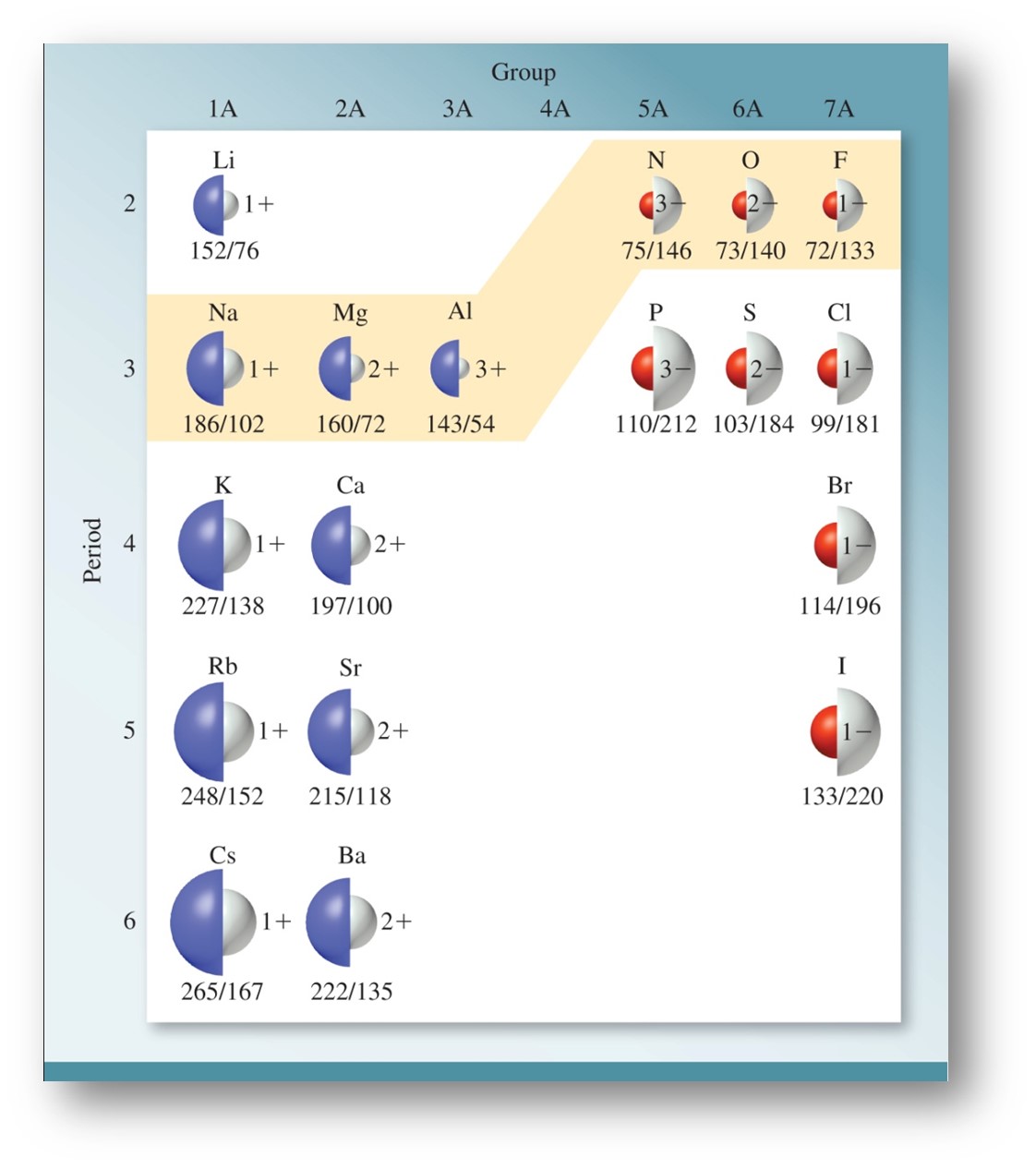
When atoms and ions share an electron configuration, they are said to be isoelectronic. For the three species, O
The next trend is ionization energy, which increases from left to right and bottom to top across the periodic table. Ionization energy (IE) is the minimum energy required to remove an electron from an atom in the gas phase, which results in the creation of an ion. This trend is shown in the image below.

In general, as the effective nuclear charge increases, ionization energy also increases. More specifically, within a given shell, electrons with a higher value of the angular momentum quantum number (l) are higher in energy and thus, easier to remove. This can be seen in the following image.
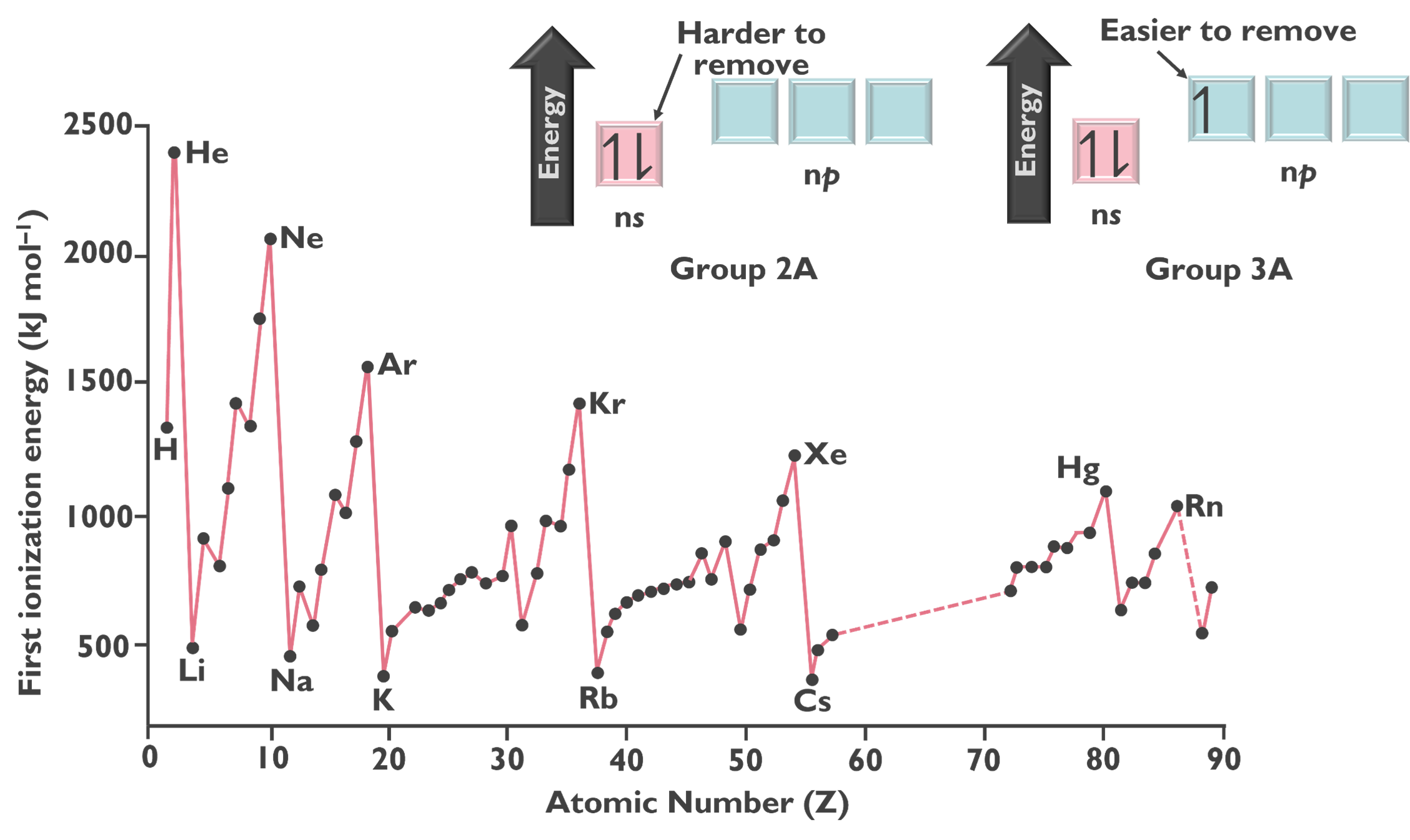
Additionally, removing a paired electron is easier because of the repulsive forces between two electrons in the same orbital.
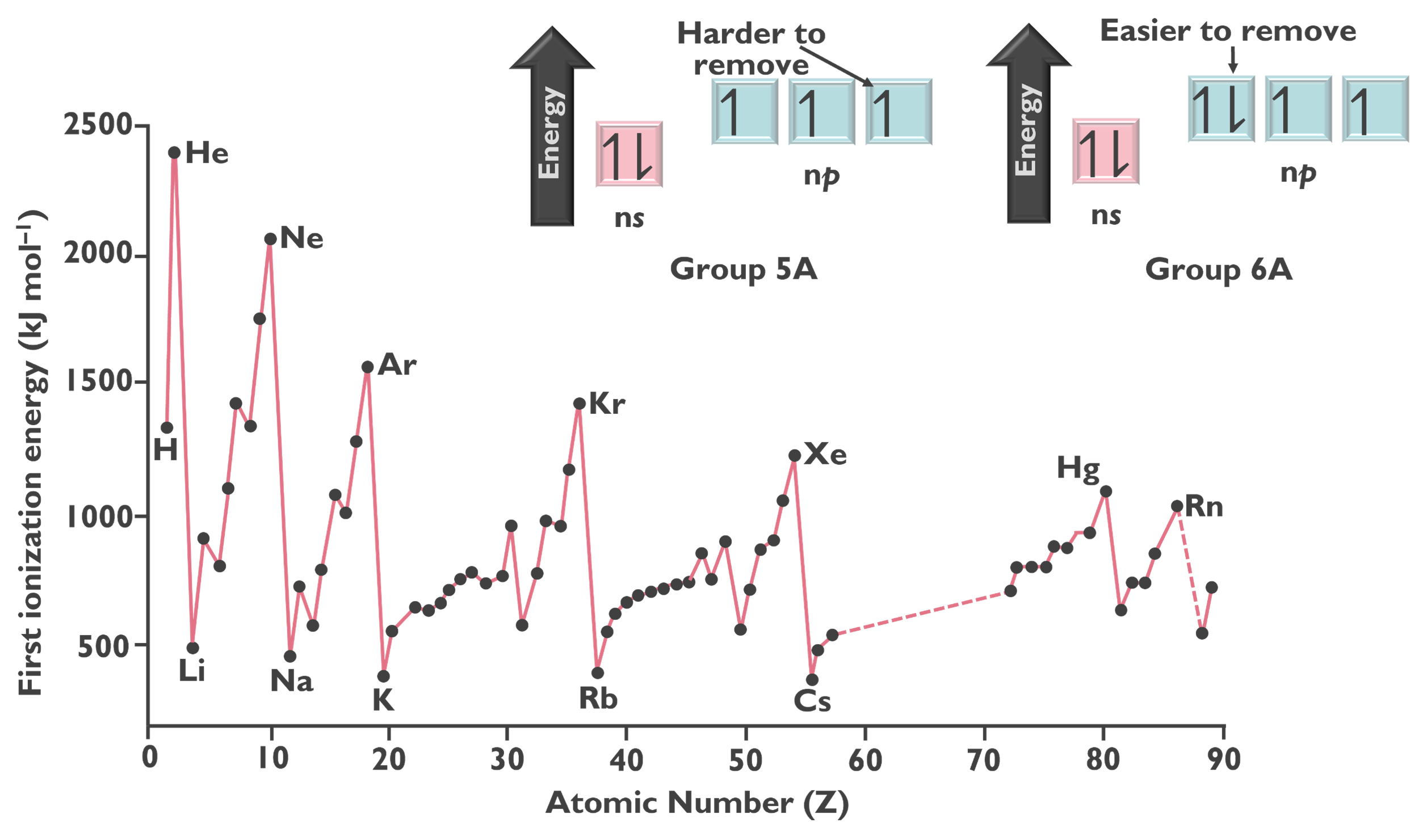
Energy is required to remove an electron, making this ionization an endothermic process, leading to positive ionization energy values. There is an increase in energy with each ionization, the second ionization even, third ionization event, fourth ionization event, etc. This can be attributed to there being a stronger electrostatic attraction between a cation and electron.
The last trend to discuss is electron affinity (EA), which is the energy change for the process of adding an electron to a gaseous atom to form an anion. This process can be either endothermic (requiring energy) or exothermic (releasing energy). This trend increases from left to right across a period as Zeff increases.
The trends discussed in this chapter can be summarized in the following image.

Practice – Periodic Trends
As one moves left to right across the periodic table, how do the following
atomic properties generally change?
i. atomic radius
ii. electronegativity
iii. electron affinity
- decreases, decreases, increases
- increases, increases, decreases
- increases, increases, increases
- decreases, increases, increases
Solution
Answer: D
Practice – Periodic Trends
As one moves down the periodic table, how do the following
atomic properties generally change?
i. atomic radius
ii. electronegativity
iii. electron affinity
- decreases, decreases, increases
- increases, decreases, decreases
- increases, increases, increases
- decreases, increases, increases
Solution
Answer: B