5.1 Practice Problems
Attempt these problems as if they were real exam questions in an exam environment.
Only look up information if you get severely stuck. Never look at the solution until you have exhausted all efforts to solve the problem. Having to look up information or the solution should be an indicator that the previous layers (1–5) in the Structured Learning Approach have not been mastered.
What is the hybridization of all the C atoms in the molecule below?
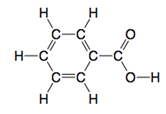
- sp
- sp3
- sp4
- sp2
- sp2d2
Solution
Answer: D
Concept: Hybridization
Which statement is not correct regarding the sulfur hybridization in the following species?
H2S SO3 SO32– SO42– SF4- sp3 hybrid in SO32–
- sp3 hybrid in SO42–
- sp3 hybrid in H2S
- sp2 hybrid in SO3
- sp3 hybrid in SF4
Solution
Answer: E
Concept: Hybridization
How many sigma and pi bonds are in benzoic acid?

- 15 sigma bonds and 4 pi bonds
- 11 sigma bonds and 8 pi bonds
- 11 sigma bonds and 4 pi bonds
- 15 sigma bonds and 8 pi bonds
- 11 sigma bonds and 7 pi bonds
Solution
Answer: A
Concept: Sigma and pi bonding
Predict if the B2 molecule is paramagnetic or diamagnetic and identify the bond order using the given molecular orbital diagram.
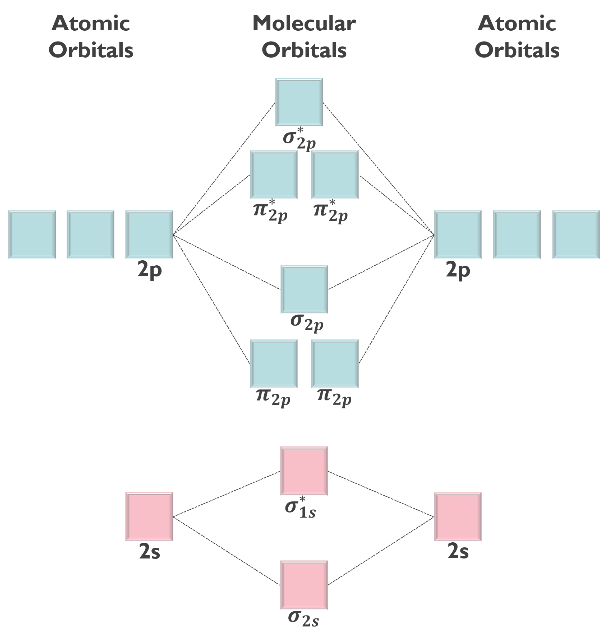
- paramagnetic; bond order = 2
- paramagnetic; bond order = 0.5
- diamagnetic; bond order = 2
- paramagnetic; bond order = 1
- diamagnetic; bond order = 1
Solution
Answer: D
Concept: MO diagrams