4.1 Ionic Compounds
A three-dimensional array of oppositely-charged ions is called a **l*attice. Lattice energy** is the amount of energy required to convert a mole of ionic solid to its constituent ions in the gas phase.
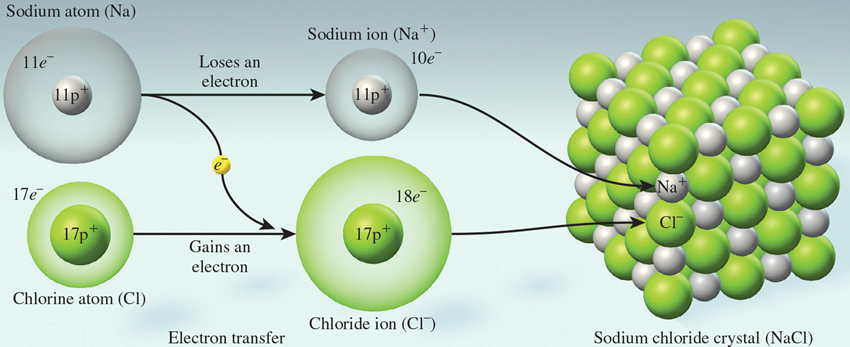
Figure 4.3: Ionic bonding in a lattice
The formation of ionic bonds releases a large amount of energy
The magnitude of lattice energy is a measure of an ionic compound’s stability. Lattice energy depends on the magnitudes of the charge and on the distance between them.
\[F \propto \dfrac{Q_1 \times Q_2}{d^2}\] where Q is the amount of charge and d is the distance separating those charges.
The magnitude of lattice energy is a measure of an ionic compound’s stability. Lattice energy depends on the magnitudes of the charge and on the distance between them.
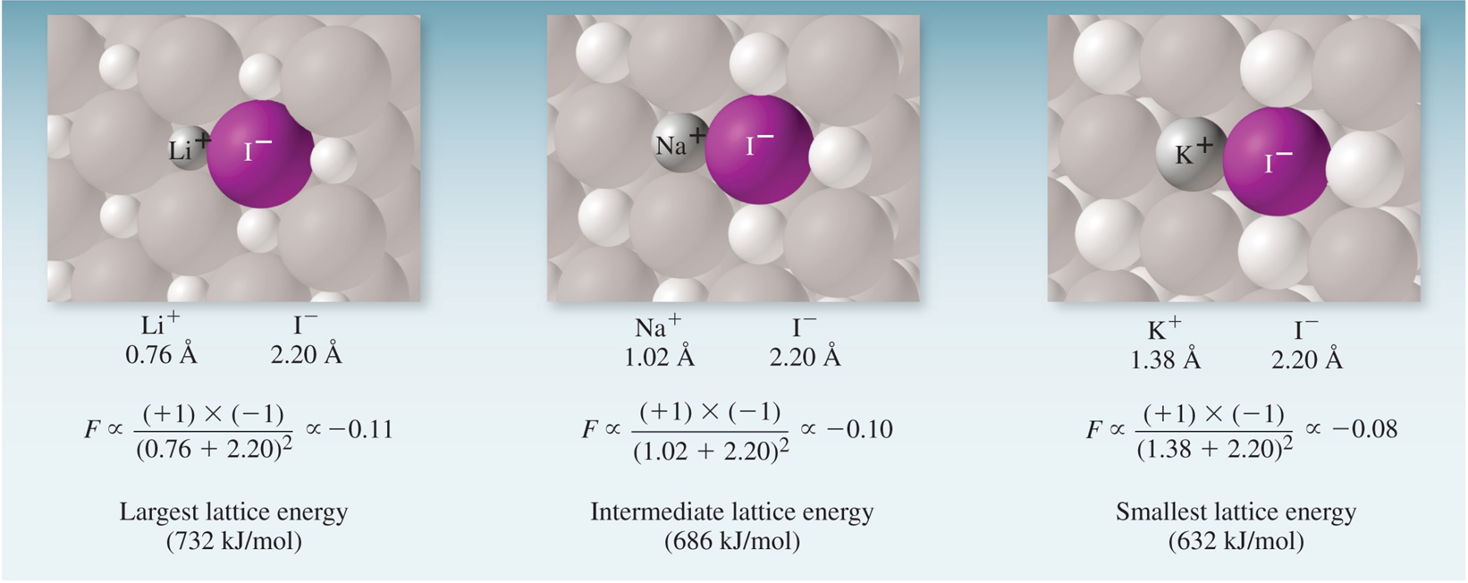
Figure 4.4: Ionic bonding in a lattice
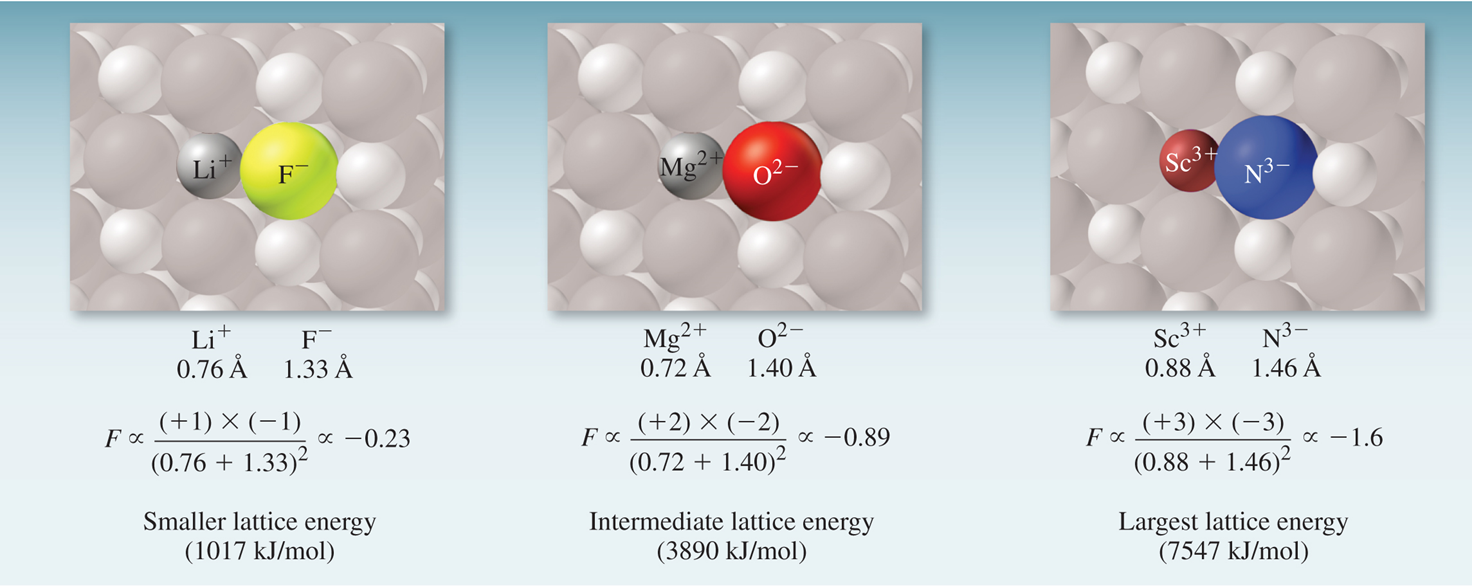
Figure 4.5: Ionic bonding in a lattice
| Compound | Lattice Energy (kJ/mol) | Melting Point (°C) |
|---|---|---|
LiF |
1017 |
845 |
LiCl |
860 |
610 |
LiBr |
787 |
550 |
LiI |
732 |
450 |
NaCl |
787 |
801 |
KCl |
699 |
772 |
MgO |
3890 |
2800 |
ScN |
7547 |
|
4.1.1 Electron Configurations of Ions
To write the electron configuration of an ion formed by a main group element:
- Write the configuration for the atom.
- Add or remove the appropriate number of electrons. Positive ions, remove electrons; negative ions, add electrons
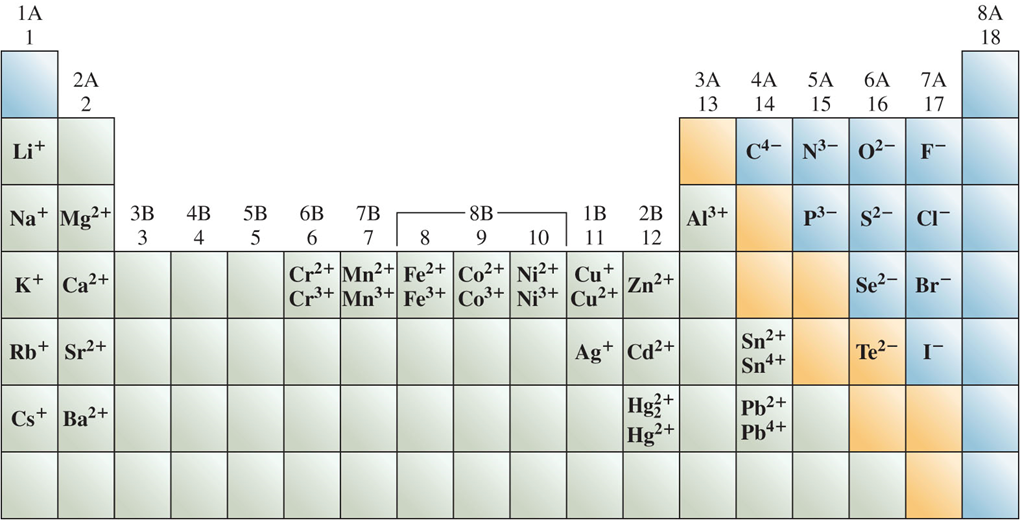
Figure 4.6: Common ions of the elements
Species with identical electron configurations to the noble gas to the right are called isoelectronic.
Common monatomic ions arranged by their positions in the periodic table
- Note that mercury(I) is a polyatomic ion (Hg22+)
Practice – Charge Notation
Write electron configurations for the following ions of main group elements:
- N3–
- Ba2+
- Be2+
Solution
- [He]2s22p6
- [Kr]5s4d10105p6
- 1s2
Practice – Isoelectronic
Which of the following ions are isoelectronic?
- Cl–
- N–3
- K+
- Ca2+
Solution
Cl–, K+, Ca2+
Practice – Charge Notation
Which of the following notations is incorrect?
- Li+Cl–
- Hδ+Clδ–
- C+O–
- all of the above are incorrect
- only (b) and (c) are correct
Solution
Answer: C
Carbon monoxide, CO, is not ionic and the notation should only use partial (δ) charges.
4.1.2 Ions of the d-Block Elements
Ions of d-block elements are formed by removing electrons first from the shell with the highest value of n.
For Fe to form Fe2+, two electrons are lost from the 4s subshell not the 3d.
Fe: [Ar]4s23d6 → Fe2+: [Ar]3d6
Fe can also form Fe3+, in which case the third electron is removed from the 3d subshell.
Fe: [Ar]4s23d6 → Fe3+: [Ar]3d5
4.1.3 Electronegativity and Polarity
There are two extremes in the spectrum of bonding:
- covalent bonds occur between atoms that share electrons
- ionic bonds occur between a metal and a nonmetal and involve ions
Bonds that fall between these extremes are polar.
In polar covalent bonds, electrons are shared but not shared equally. The delta, δ, is used to denote partial charges on the atoms

Electron density maps show the distributions of charge. Electrons spend a lot of time in red and very little time in blue.
Electronegativity is the ability of an atom in a compound to draw electrons to itself. Electronegativity varies with atomic number.
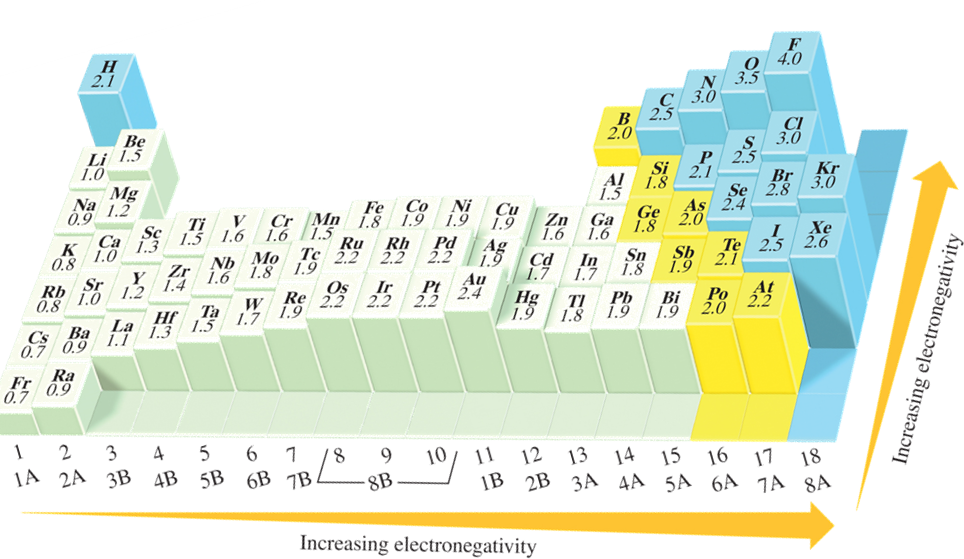
There is no sharp distinction between nonpolar covalent and polar covalent or between polar covalent and ionic.
The following rules help distinguish among them:
A bond between atoms whose electronegativites differ by less than 0.5 is general considered purely covalent or nonpolar.
A bond between atoms who’s electronegativies differ by the range of 0.5 to 2.0 is generally considered polar covalent.
A bond between atoms whose electronegativities differ by 2.0 or more is generally considered ionic.
In polar covalent bonds, electrons are shared but not shared equally. The delta, δ, is used to denote partial charges on the atoms
Electron density maps show the distributions of charge. Electrons spend a lot of time in red and very little time in blue.

An arrow is used to indicate the direction of electron shift in polar covalent molecules.

The consequent charge separation can be represented as Delta (&delta) which denotes a partial positive or negative charge.

Practice – Electron Configuration
Elements A, B, and C have electronegativities of 3.5, 2.5, and 0.2, respectively. Which of the following bonds would be considered polar covalent?
- A–A
- A–B
- A–C
- B–C
- none of the above
Solution
Answer: B
Recall that bonds can be classified by the difference in the electronegativities of the atoms involved in that bond.
- < 0.5 → pure covalent
- 0.5 to 2.0 → polar covalent
- > 2.0 → ionic
Therefore,
- A–A is pure covalent (nonpolar): 3.5 - 3.5 = 0
- A–B is polar covalent: 3.5 - 2.5 = 1.0
- A–C is ionic: 3.5 - 0.2 = 3.3
- B–C is ionic: 2.5 - 0.2 = 2.3
4.1.4 Dipole Moment, Partial Charges, and Percent Ionic Character
A quantitative measure of the polarity of a bond is its dipole moment (μ).
\[\mu = Q \times r\] where μ is always positive and expressed in debye units (D), Q is the charge, and r is the distance between the charges.
Note: 1 D = 3.336×10–30 C m. C is the Coulomb and m is the meter
| Molecule | Bond Length (Å) | Dipole Moment (D) |
|---|---|---|
HF |
0.92 |
1.82 |
HCl |
1.27 |
1.08 |
HBr |
1.41 |
0.82 |
HI |
1.61 |
0.44 |
Although the designations “covalent,” “polar covalent,” and “ionic” can be useful, sometimes chemists wish to describe and compare chemical bonds with more precision.
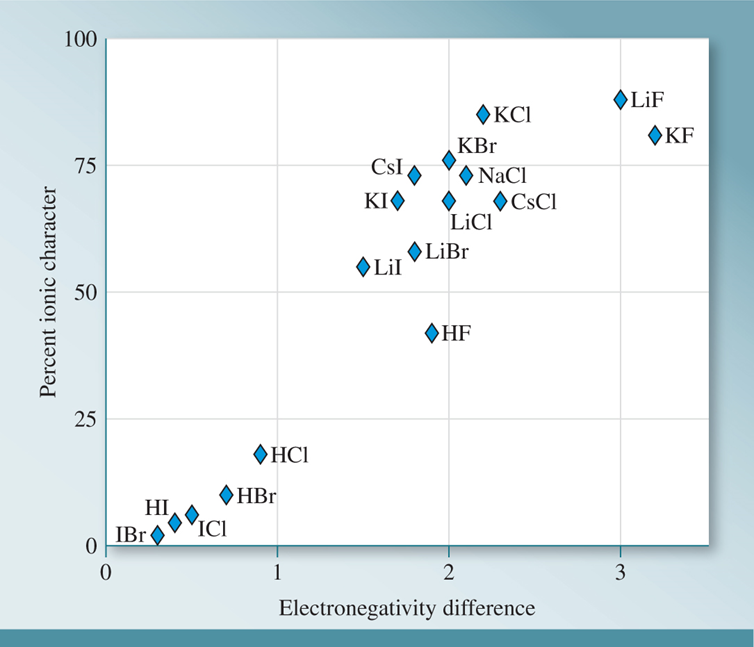
Comparing the calculated dipole moment with the measured values gives us a quantitative way to describe the nature of a bond using the term percent ionic character.
\[\%~\mathrm{ionic~character} = \dfrac{\mu~\mathrm{(observed)}}{\mu~\mathrm{(calculated~ assuming~discrete~charges)}} \times 100\%\] The figureb below demonstrates the relationship between percent ionic character and the electronegativity difference in a heteronuclear diatomic molecule.
4.1.5 Types of Ionic Compounds
There are two types of ionic compounds:
- Ionic compounds formed between two elements: a metal and a non-metal
- NaCl, CaCl2
- Ionic compound formed between a metal and a polyatomic ion (multiple atoms)
- Ca(BrO3)2
The list of polyatomics is given in your textbook, but in this case, the polyatomic ion is the bromate ion (BrO3). The polyatomic ions must be memorized.
Naming ionic compounds composed of only elemental ions requires you to know the names and symbols of the given metal and non-metal ions.
These are given in Table 5.2 of your book. I will also give uploaded notes with them.
To name ionic compounds:
- Name the cation (omit the word ion)
- Use a Roman numeral if the cation can have more than one charge (this happens mostly for transition metals).
- This roman numeral tells the charge on that transition metal.
- Name the anion by replacing the ending with -ide (omit the word ion)
Examples:
- NaBr, sodium bromide
- FeCl2, iron (II)chloride (old: ferrous chloride)
- FeCl3, iron (III)chloride (old: ferric chloride)
To give ionic compound formula from the name:
When we want to go from the name to the formula there are a few things we need to remember. Ionic compounds are electronically neutral.
For ionic compounds to be electronically neutral, the sum of the charges in each formula must be **zero*.**
Your task: Find the smallest coefficients that will yield a compound with a neutral charge. People accomplish this one of two ways: (1) Sum of Charges or (2) Cross Multiplication
- Aluminum oxide: Al3+ and O2– → Al2O3
The sum of the charges are 2(+3) + 3(–2) = 0
4.1.6 Formulas of Ionic Compounds
If you chose to cross multiply, make sure that you are only reporting the empirical formula. What do I mean by that?
Example: What is the formula for Calcium Oxide?
Order to writing the chemical formula:
- Look for Calcium (Ca) on the periodic table: It is in group 2A (typical ion charge of 2+).
- Find Oxygen (O) on the periodic table: It is group 6A (typical ion charge of 2–).
- We write Ca with a +2 charge. We write O with a –2 Charge.
- We cross multiply
- We divide to make the simplest coefficients possible.
4.1.7 Ionic Compounds formed with polyatomic ions (covalently bonded species)
Polyatomic ions: consist of a combination of two or more atoms. A common list can be found in the textbook in Table 2.5.
Formulas are determined following the same rules as for ionic compounds containing only monatomic ions: ions must combine in a ratio that give a neutral formula overall.
4.1.8 Polyatomic Ions (Oxoanions)
Oxoanions are polyatomic anions that contain one or more oxygen atoms and one atom (the “central atom”) of another element.
Starting with the oxoanions that end in –ate, we can name these ions as follows:
- The ion with one more O atom than the –ate ion is called the per…ate ion. Thus, ClO3– is the chlorate ion, so ClO4– is the perchlorate ion.
- The ion with one less O atom than the –ate ion is called the –ite ion. Thus, ClO2– is the chlorite ion.
- The ion with two fewer O atom than the –ate ion is called the hypo…ite ion. Thus, ClO– is the hypochlorite ion.
You can apply these guidelines when necessary.
