2.1 Evolution of Atomic Theory
2.1.1 Dalton’s Atomic Theory

Figure 2.1: John Dalton (1803-1807)
- All matter is composed of tiny particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change.
- All atoms of a given element have identical chemical properties that are characteristic of that element.
- A compound consists of atoms of 2 or more elements combined in a small, whole-number ratio.
- Atoms can change how they are combined, but they are neither created nor destroyed in chemical reactions.
The Law of Conservation of Mass states that there is no detectable change in mass during an ordinary chemical reaction The Law of Constant Composition/Law of Definite Proportion states that one chemical sample will be the same as another chemical sample A chemical compound always contains the same elements in the same proportions by mass Aspirin will always have 9 Carbon, 8 Hydrogen and 4 Oxygen Atoms
Figure 2.2: John Dalton (1803-1807)
The Law of Multiple Proportions (Dalton’s Law) states that When two elements react, the mass of one element will react with masses of a second element so compounds always equals a ratio of small, whole number.
Compound A
32 grams of O reacts with 24 grams of C
\[\mathrm{ratio} = \dfrac{\mathrm{O}}{\mathrm{C}} = \dfrac{32}{24} = 1.33\]
Compound B
64 grams O reacts with 24 grams of C
\[\mathrm{ratio} = \dfrac{\mathrm{O}}{\mathrm{C}} = \dfrac{64}{24} = 2.66\]
\[\dfrac{\mathrm{Compound~A}}{\mathrm{Compound~B}} = \dfrac{2.66}{1.33} = \dfrac{2}{1}\]
Compound B has 2 O for every C; compound A has 1 O for every C.
2.1.2 Thomson
In the late 1800’s, many scientists were doing research involving radiation, the emission and transmission of energy in the form of waves. They commonly used a cathode ray tube, which consists of two metal plates sealed inside a glass tube from which most of the air has been evacuated.
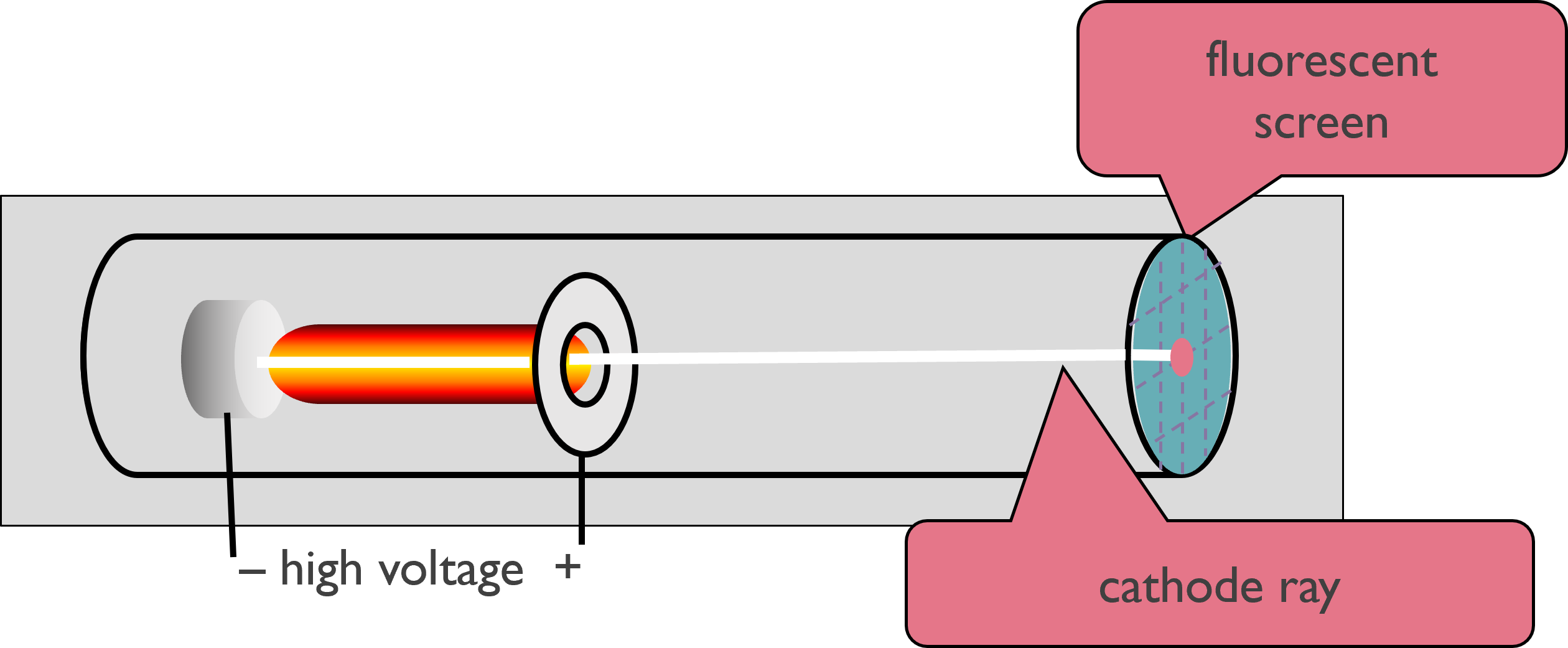
Researchers discovered that like charges repel each other, and opposite charges attract one another. J. J. Thomson (1856–1940) noted the rays were repelled by a plate bearing a negative charge and attracted to a plate bearing a positive charge.

Listen to J.J. Thompson talking about an electron
J. J. Thompson
- proposed the rays were a stream of negatively charged particles and these are called electrons
- determined the charge–to–mass ratio of electrons to be 1.76×108 C g–1
- proposed the “plum pudding” model of an atom (“+” and “–” charges are squished together like a chocolate chip cookie)
Electric and magnetic fields deflect the beam.
- Gives charge/mass of e– = 1.76×108 C g–1
- Coulomb (C) = SI unit of charge
- 1 C = A·s
2.1.3 Millikan
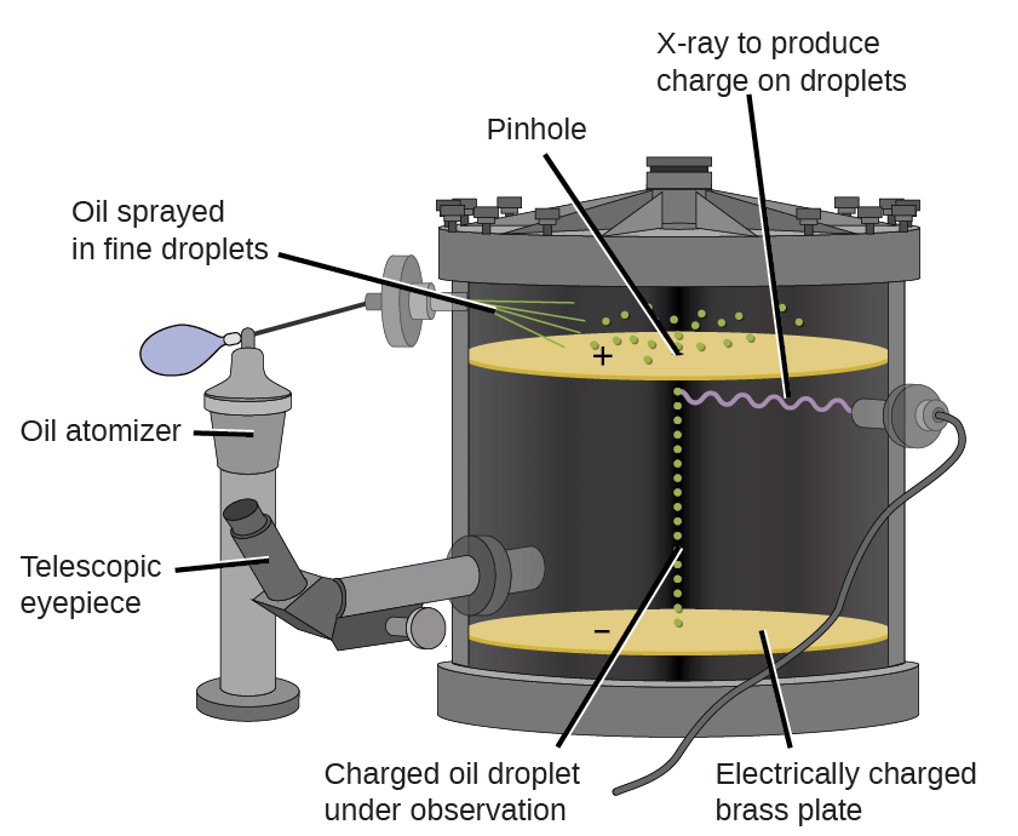
R. A. Millikan (1868–1953) determined the charge on an electron by examining the motion of tiny oil drops that were electrically charged. The charge of an electron was determined to be –1.6022×10–19 C.
2.1.4 Rutherford
Ernest Rutherford used α particles to find the structure of atoms. Most particles penetrated the gold foil undeflected, but some were deflected at a large angle, while others bounced back in the direction from which they had come.

Rutherford proposed the nuclear model where the positive charge is concentrated in the nucleus. The nucleus accounts for most of an atom’s mass (extremely dense central core in the atom).
- A typical atomic radius is about 100 pm
- A typical nucleus has a radius of about 5×10–3 pm
- 1 pm = 1×10–12 m