1.1 Phases and Classification of Matter
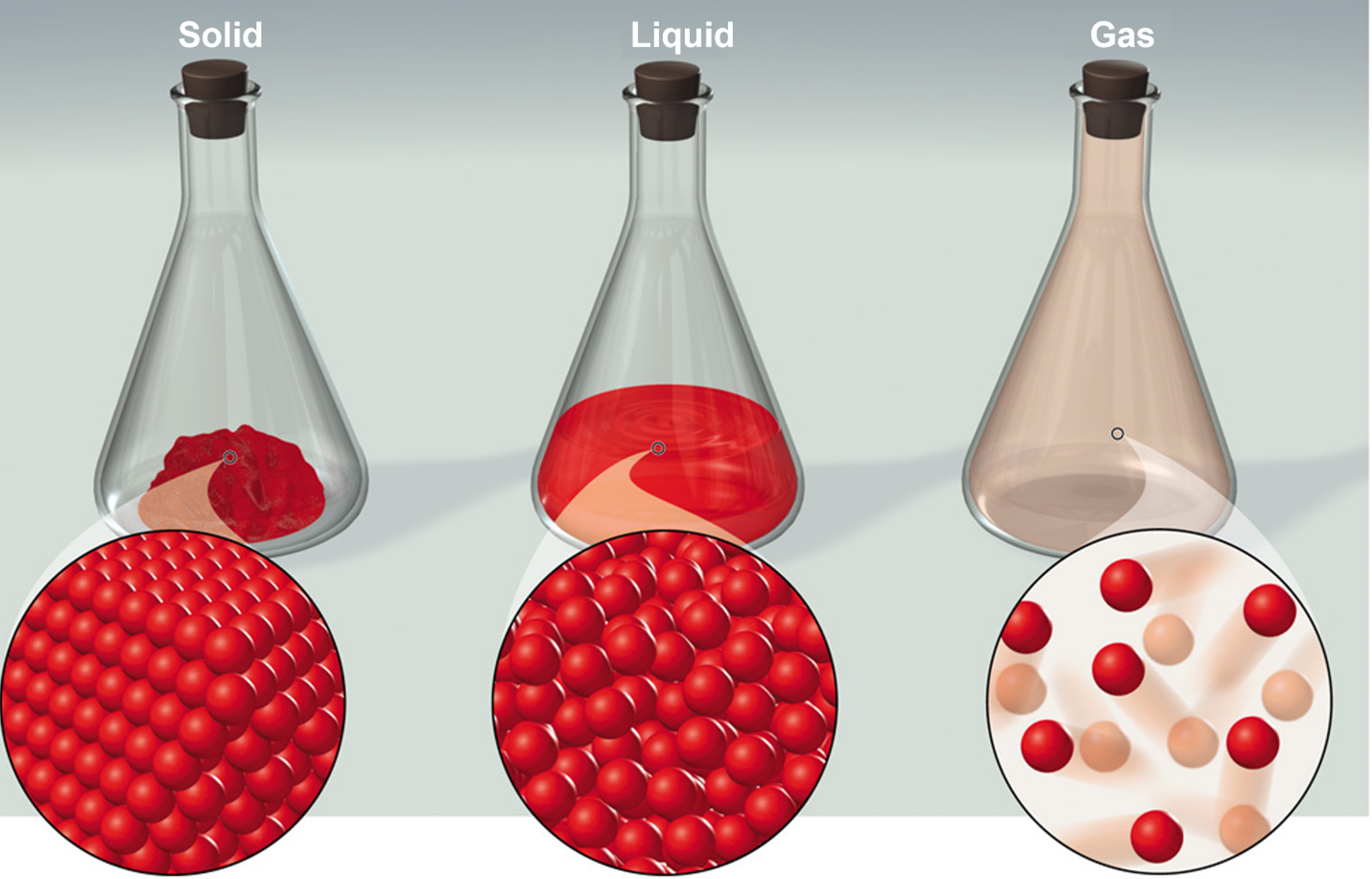
In terms of matter the three main forms that we’re going to talk
about in this class are solids liquids and gases. Solids are
uniformed in their structure on the molecular level. So, on the
micro scale (the molecular level) we see there’s very little space
between the different molecules or atoms in that solid. Solids have
a fixed volume and shape.
In terms of liquids on the micro scale, we see is a greater distance
between the different molecules or atoms. Liquids have a fixed
volume, and its shape will conform to the container that it is
placed in.
Gases on the other hand have an even greater distance between the
different molecules or atoms. When we put a gas into a container the
gas is going to take the shape of the container and it will also
take the volume of the container.
There is a fourth state of matter, plasma. This state of matter
naturally occurs in the interiors of stars (as well as other
high-temperature environments) and it is a gaseous state that
contains an appreciable number of electrically charged particles.
The law of conservation of matter states that there is no detectable change in the total quantity of matter present when matter converts from one type to another (a chemical change) or changes between phases: solid, liquid, or gas (a physical change).
We can classify matter as either a pure substance or a mixture of substances. A pure substance is a form of matter that has definite composition and distinct properties, and pure substances can be broken into two categories: elements and compounds. Elements are substances that cannot be broken down into simpler substances and compounds are comprised or two or more elements. Compounds can therefore be broken down by chemical changes, into elements or other compounds.
A mixture is a physical combination of two or more substances. We can have what’s called a homogeneous mixture or heterogeneous mixture. The difference between these two, a homogeneous is uniform throughout. In a heterogeneous mixture you can see the different components of that mixture. In the image below we have matter, it could be a solid liquid or gas. It’s just anything that has mass and takes up space.
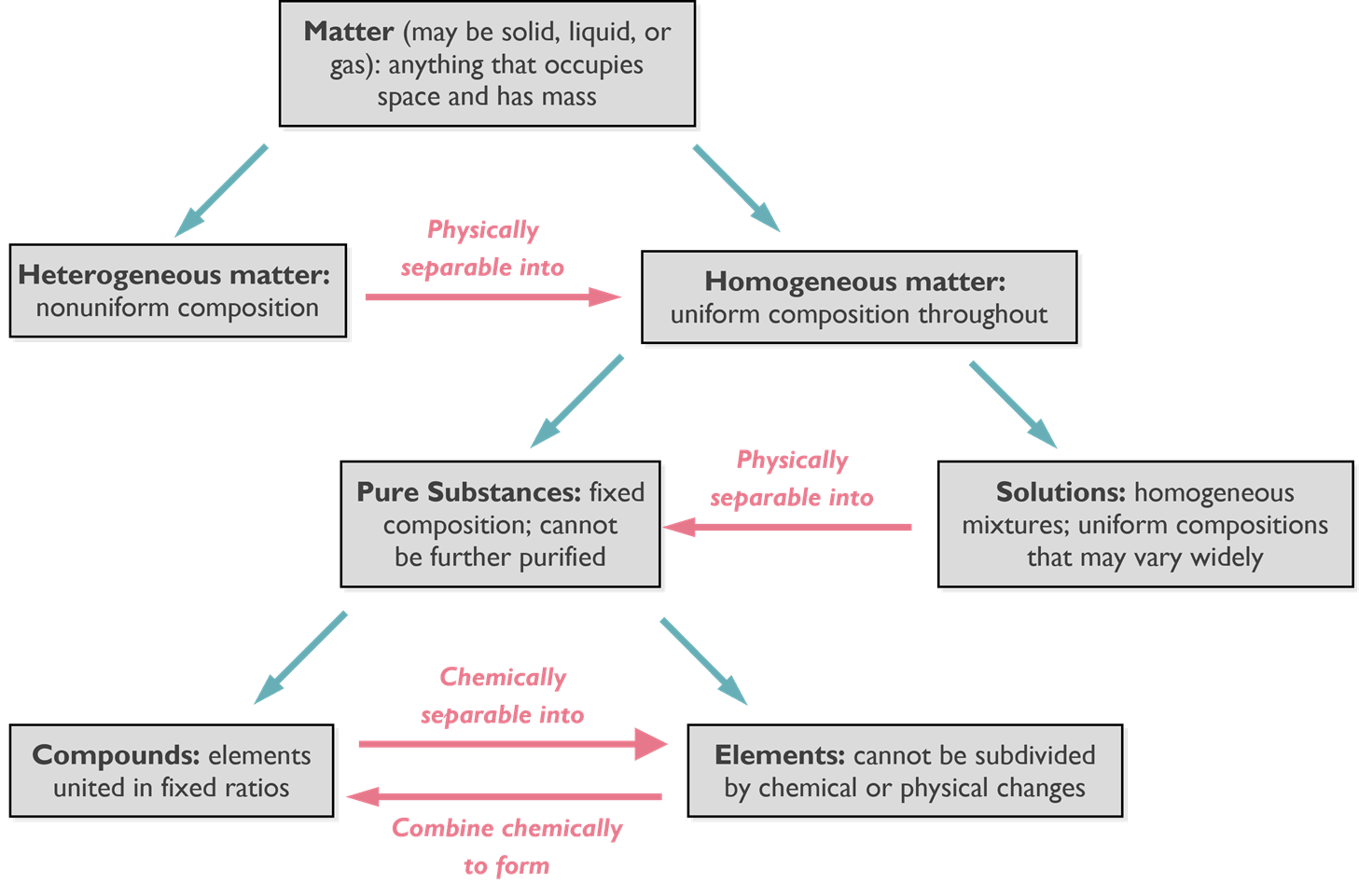
The first thing we must identify is what kind of matter it is: heterogeneous matter, non-uniform composition, or homogeneous matter. If it’s heterogeneous matter, we know that we can pull the different components out to make it homogeneous matter. Once we have homogeneous matter or if it’s homogeneous to start with, we have two possibilities. Either you have a pure substance, or you have a solution. If we have a solution, we can separate the uniform components using techniques like liquid chromatography. Once we’ve separated the solution we are left with a pure substance. We have two options with the pure substance. We either have a compound or an element. If it is a compound, we can break this down a little further. A compound is composed of elements in fixed ratios. We can go from a compound to the elements through a chemical reaction to separate that compound into various elemental forms.
An atom is the smallest particle of an element that has the properties of that element and can enter a chemical combination. In other words, an atom is the smallest piece of an element that still retains those elements properties. Molecules consist of two or more atoms joining together through chemical bonds. Molecules can be composed of two of more identical atoms (homonuclear) or they can be composed of two of more different atoms (heteronuclear).
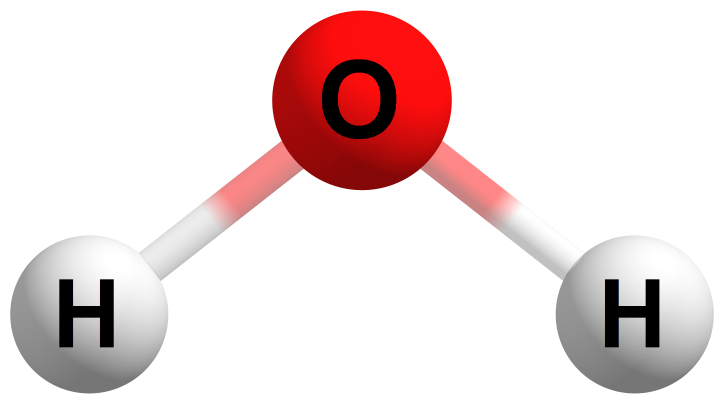
Below is an example of a water molecule which consists of three atoms bonded together: two hydrogen (H) atoms and one oxygen (O) atom.
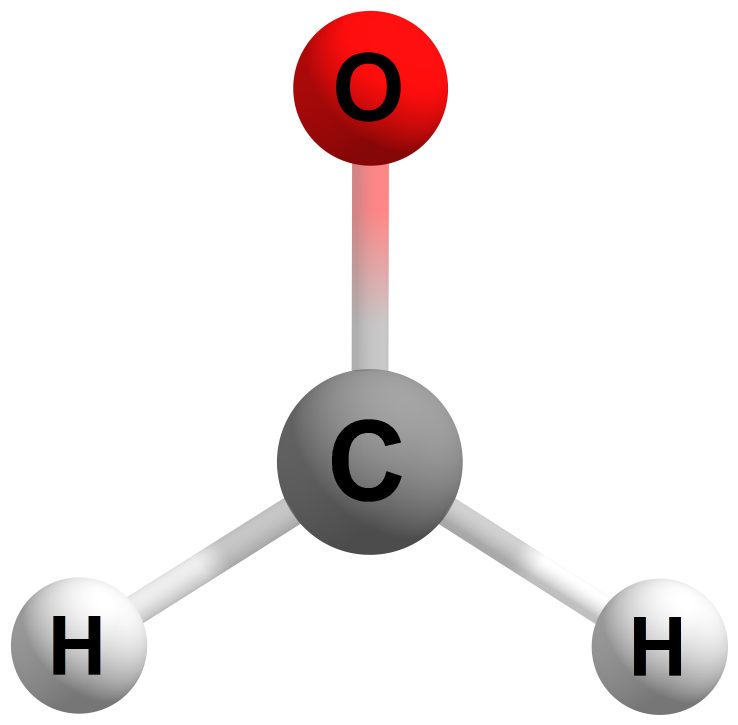
The formaldehyde molecule contains four atoms: one carbon (C) atom, two hydrogen (H) atoms, and one oxygen (O) atom.