2.4 Average Atomic Mass
Most elements occur as a mixture of isotopes. Consider a sample of magnesium. The sample would contain a mixture of stable magnesium isotopes.
| 24Mg | 25Mg | 26Mg | |
|---|---|---|---|
Number of p+ |
12 |
12 |
12 |
Number of n |
12 |
13 |
14 |
Mass (amu) |
23.985 |
24.986 |
25.982 |
Atomic mass is the mass of an atom in atomic mass units (amu). The average atomic mass on the periodic table represents the average mass of the naturally occurring mixture of isotopes.
| Isotope | Isotopic Mass (amu) | Natural Abundance (%) |
|---|---|---|
12C |
12.00000 |
98.93 |
13C |
13.003355 |
1.07 |
The most direct and most accurate method for determining atomic and molecular masses is mass spectrometry, using a mass spectrometer.
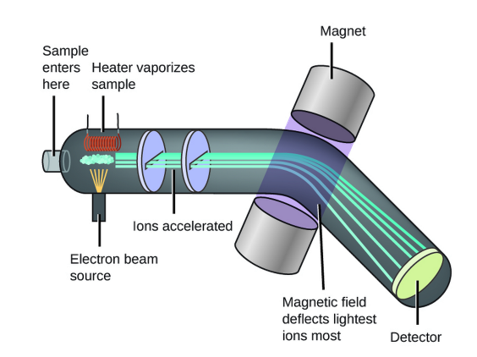
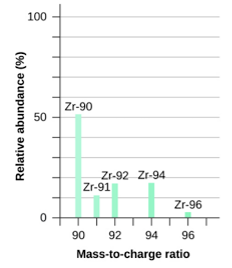
Practice
Chlorine has two stable isotopes, 35Cl (75.78%) and 37Cl (24.22%) which have masses of 34.9689 and 36.9659 amu, respectively. Calculate the average atomic mass (in amu) of chlorine.
Solution
\[(0.7578)(34.9689~\mathrm{amu}) + (0.2422)(36.9659~\mathrm{amu}) = 34.44518022 = 35.45~\mathrm{amu}\]
Practice
Silver has two stable isotopes; Ag-107 (106.90509 amu) and Ag-109 (108.90476 amu). Determine the percentage abundance of these two isotopes of silver.
Solution
107Ag: 51.8%
109Ag: 48.2%
Practice
Rubidium has two naturally occurring isotopes, 85Rb (relative mass 84.9118 amu) and 87Rb (relative mass 86.9092 amu). If rubidium has an average atomic mass of 85.47 amu, what is the percent abundance of each isotope?
A. 85Rb: 52.45%; 87Rb: 47.55%
B. 85Rb: 26.81%; 87Rb: 73.19%
C. 85Rb: 72.05%; 87Rb: 27.95%
Solution
C