2.2 Thomson
In the late 1800’s, many scientists were doing research involving radiation, the emission and transmission of energy in the form of waves. They commonly used a cathode ray tube, which consists of two metal plates sealed inside a glass tube from which most of the air has been evacuated.
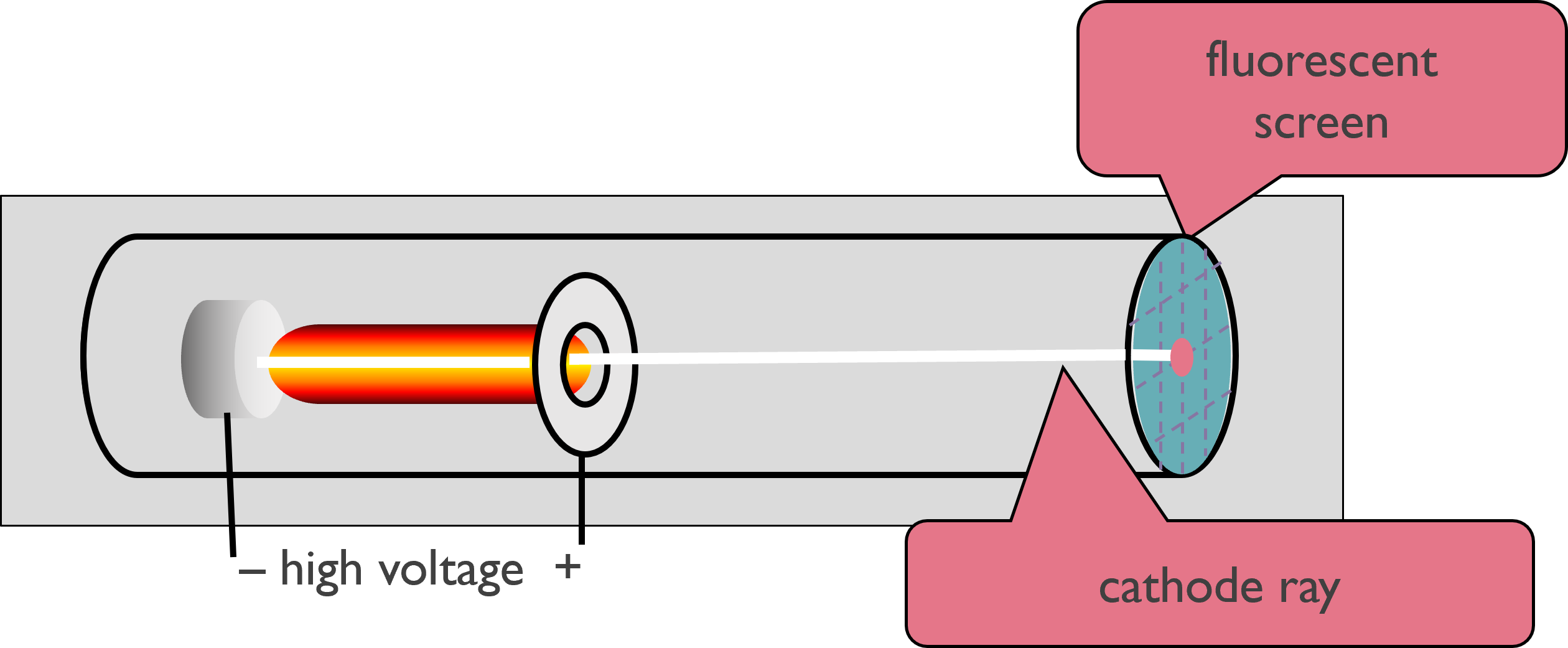
Researchers discovered that like charges repel each other, and opposite charges attract one another. J. J. Thomson (1856–1940) noted the rays were repelled by a plate bearing a negative charge and attracted to a plate bearing a positive charge.

Listen to J.J. Thompson talking about an electron
J. J. Thompson
- proposed the rays were a stream of negatively charged particles and these are called electrons
- determined the charge–to–mass ratio of electrons to be 1.76×108 C g–1
- proposed the “plum pudding” model of an atom (“+” and “–” charges are squished together like a chocolate chip cookie)
Electric and magnetic fields deflect the beam.
- Gives charge/mass of e– = 1.76×108 C g–1
- Coulomb (C) = SI unit of charge
- 1 C = A·s